Urinary Incontinence FAQs
You’ve got questions, and we’ve got answers
What is Stress Urinary Incontinence?
Stress Urinary Incontinence is the involuntary loss of urine
during physical activities, like laughing, jumping, sneezing
or lifting heavy objects. It’s the most common form of
urinary incontinence.
What are some of the symptoms of Stress Urinary Incontinence and how is it caused?
Stress Urinary Incontinence is the involuntary loss of urine
during physical activity, which may include but is not limited to: coughing, laughing or lifting. Incontinence occurs when the muscles that support the urethra (the tube that carries urine out of the body) are weakened or damaged. This can happen as a result of childbirth, trauma, obesity, family history, hormone changes and many other reasons.
My doctor says I have Stress Urinary Incontinence.
Are there different types?
One type is called hypermobility, “hyper” means too much
and “mobility” refers to movement, which can result from
childbirth, previous pelvic surgery or hormonal changes.
Hypermobility occurs when the normal pelvic floor muscles can no longer provide the necessary support to the urethra. This may lead to the urethra dropping when any downward pressure is applied, resulting in involuntary leakage. Another type is called intrinsic sphincter deficiency, also sometimes referred to as ISD. This refers to the weakening of the urethral sphincter muscles or closing mechanism. As a result, the sphincter does not function normally regardless of the position of the bladder neck or urethra.
What are some treatment options?
Many times, conservative treatment options for stress urinary incontinence are used initially. Some of those treatment options include behavioral modification — such as decreasing fluid intake, timed voiding and eliminating caffeine, or pelvic floor muscle training such as kegel exercises to strengthen the pelvic floor and sphincter muscles. These types of treatments may or may not improve symptoms. When symptoms are more severe, or conservative options aren’t working, bulking agent injections or surgery may be an option. Stress urinary incontinence can be treated in several ways, depending on the exact nature of the incontinence and its severity. As disease state and anatomy differ for each patient, outcomes may vary. Consult a specialist for all available treatment options.
What are the potential risks and complications of surgery?
As with most surgical procedures, there are potential risks
and complications associated with SUI mid-urethral sling
surgery. Your specialist can further explain your specific risks based on your medical history and surgical approach used.
Will a mid-urethral sling cure my incontinence
symptoms with 100% certainty?
There is no surgery for incontinence that has a 100% cure
rate, but mid-urethral slings for bladder leakage have been studied since the mid-1990s and have shown to have high success rates of 80-95% at one year follow-up.1-5
What is a mid-urethral sling system?
A mid-urethral sling system is designed to provide a
hammock of support under the urethra to prevent it from
dropping during physical activity.
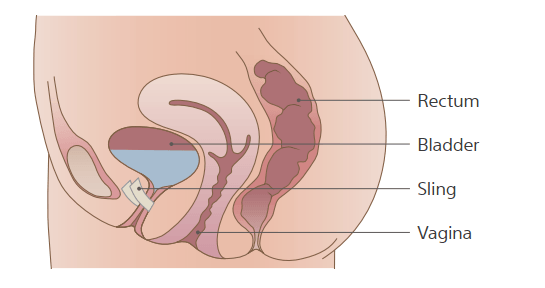
What are the types of sling options?
Many surgical options have been developed, the difference being how the mesh material is placed under the urethra. As disease state and anatomy differ for each patient, as well as the type of Stress Urinary Incontinence, consult a specialist for all available treatment options.
How do I know if a sling is a good option for me?
This is a decision that should be made by you in consultation with a specialist. You should have the opportunity to discuss with your specialist all of your treatment options, and then which treatment plan is most appropriate for your specific medical situation.
How will my surgery be performed if I elect to have a sling?
A minimally invasive sling procedure is estimated to take
between 30 to 45 minutes; this estimate can vary for many
reasons. Your specialist will discuss the type of anesthesia
with you, the specific procedure steps, and should answer all your questions. Mid-urethral sling procedures are frequently outpatient procedures, in which case, most patients return home the same day.
What can I expect during recovery?
Every patient’s recovery experience is unique, and you
should consult your specialist as to what he or she expects in your individual case. As with any surgery, it is expected that you feel some soreness but most patients return to normal activity after a short period of time.
Please consult with your surgeon on specific activities to avoid during recovery to achieve optimal outcomes. Complications associated with Boston Scientific SUI products can be found at the bottom
of this FAQ. Before you are discharged from the hospital,
you may be given a prescription for an antibiotic and/or pain medication to relieve any discomfort you may experience. You will be instructed on how to care for your incision area. At the discretion of your physician, most patients resume moderate activities within 2 to 4 weeks, with no strenuous activity for up to 6 weeks. Talk with your specialist about post-procedure complications and when to notify him or her of a potential concern.
When will I stop leaking?
Most women see results right after the procedure. Talk with your specialist about what you should expect.
Please consult your physician to discuss the associated risk and complications for the specific surgical material you receive. Below is a list of potential adverse events for Boston Scientific’s mid-urethral sling surgical material.
The following adverse events and known risks have been reported due to suburethral (beneath the urethra) mesh sling placement, any of which may be ongoing, but are not limited to:
Abscess (swollen area within the body tissue, containing a buildup of pus), Allergic reaction to the implant, Apareunia (inability to perform sexual intercourse), Bleeding from the vagina, Hematoma formation (bruising), Complete failure of the procedure/failure to resolve a patient’s stress urinary incontinence, Dehiscence of vaginal incision (opening of the
incision after surgery), De novo detrusor instability (involuntary contraction of the bladder wall leading to an urge to urinate), Dyspareunia (pain during sexual intercourse), Edema and erythema at the surgical site (swelling and redness), Fistula formation (a hole/passage that develops through the wall of the organs) that may be acute or chronic, Foreign body reaction (body’s response to the implant) that may be acute or chronic, Infection, Inflammation that may be acute or chronic (redness, heat, pain or swelling at the surgical site as a result of the surgery), Irritation (redness or pain) at surgical site, Leg weakness (muscle weakness), Mesh contracture (mesh shrinkage), Erosion into the following organs: urethra, bladder, or other surrounding tissues and exposure/extrusion into the vagina (when the mesh goes
through the vagina into other organs or surrounding tissue), Pain or discomfort to the patient’s partner during intercourse, Pain/Ongoing Pain/Severe/Chronic Pain in the pelvis, vagina, groin/thigh, and suprapubic area that may be acute or chronic (pain or ongoing pain just above the pubic bone, pelvis, vagina, groin/thigh area that may be severe and could last for a long time), Pain with intercourse that may not resolve, Perforation or laceration of vessels, nerves, bladder, urethra or bowel (a hole in or damage to these or other tissues that may happen during placement), Scarring, scar contracture (tightening of the scar), Stone formation (as a result of mesh erosion/exposure/extrusion in the urethra or bladder where the mesh is exposed to urine, mineral deposits may form along the mesh, also known
as stones), Tissue contracture (tightening of the tissue), Voiding dysfunction: incontinence,
temporary or permanent lower urinary tract obstruction, difficulty urinating, pain with urination, overactive bladder, and retention (involuntary leakage of urine or reduced or complete inability to empty the bladder from the mesh being implanted too tightly beneath the urethra). The following additional adverse events have been reported for the Solyx SIS System: Dysuria (painful/difficult urination), Hematuria (blood in the urine).The occurrence of these events may require surgical intervention and possible removal of the entire mesh. In some instances, these events may be permanent after surgery or other treatments. Removal of mesh or correction of mesh-related complications may involve multiple surgeries. Complete removal of mesh may not be possible and additional surgeries may not always fully correct the complications.
REFERENCES
1. Primus G (2006) One year follow-up on the SPARC sling system for the treatment of female
urodynamic stress incontinence. Int J Urol 13: 1410-1414
2. Andonian S et al. (2005) Randomized clinical trial comparing suprapubic arch sling (SPARC) and
tension-free vaginal tape (TVT): one-year results. Eur Urol 47: 537-54
3. Dalpiaz O et al. (2006) SPARC sling system for treatment of female stress urinary incontinence
in the elderly. Eur Urol 50: 826-830
4. Davila G et al. (2006) Multicenter experience with the Monarc transobturator sling system to
treat stress urinary incontinence. Int Urogynecol J 17:460-465
5. Tseng LH et al. (2005) Randomized comparison of suprapubic arc sling procedure vs tension-free
vaginal taping for stress incontinent women. Int Urogynecol J Pelvic Floor Dysfunct
2005 May-Jun;16(3):230-
Content provided by Boston Scientific
